Infection or disruption of the wires connecting the surgically divided sternum may lead to sternal instability and subsequent sternal separation, pain, and excessive motion of the sternal edges. As sternal non-union may lead to mediastinitis or deep sternal wound infection, early detection and management are essential.
Risk factors for developing sternal instability following median sternotomy include: large breasts, diabetes mellitus, obesity, bilateral internal mammary artery harvesting, reoperation procedures, increased blood product requirement, and excessive coughing.
Sternal stability assessment
Sternal instability can be diagnosed by real-time ultrasound or physical assessment of the sternum employing the Modified Sternal Instability Scale (SIS). The SIS is a reliable scale of sternal instability and prompts timely referral and management to the cardiac surgeon.
Clinical examination
Patients with sternal instability often describe a clicking, clunking sensation and pain with activities such as reaching, rolling over in bed and getting up out of a chair. Assessment post-surgery should note:
- Pain quality and location– intermittent/constant, dull/sharp, hot/cold, deep/superficial
- Feelings of instability or excessive motion – e.g., patient reports that their chest feels like it is going ‘fall open’
- Clicking, or clunking type sounds
- Activities that provoke symptoms of pain, clicking or crepitus
- Wound or scar status including the colour, sensitivity to temperature, discharge (serous or coloured), hypersensitivity, and presence of keloid scarring or adhesions.
Modified Sternal Instability Scale (SIS) assessment procedure:
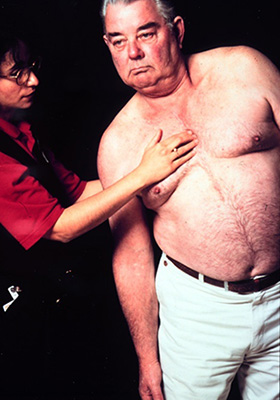
Note the grade of sternal instability when palpating the sternum during movement (see table below for definitions of grades). Palpate the upper and lower regions of the sternum separately as the lower sternum is often more unstable than the upper region. Palpate between the sternal halves using the 2nd, 3rd, and 4th digits (as shown in the figure below) during the following movements:
- Shoulder flexion (unilaterally and/or bilaterally)
- Trunk lateral flexion and/or rotation
- Coughing and deep inspiration and expiration
Select the grade that corresponds with the findings of the physical examination.
Ideally, all patients should undergo a sternal stability assessment at 3 to 5 days post-surgery, at the commencement of cardiac rehabilitation (3 to 6 weeks post-surgery), and prior to commencing advanced upper limb stretches and resistance exercises.
| Sternal Instability |
Description |
| Grade 0 |
Clinically stable sternum (no detectable motion) – normal |
| Grade 1 |
Minimally separated sternum (slight increase in motion) |
| Grade 2 |
Partially separated sternum – regional (moderate increase in movement) |
| Grade 3 |
Completely separated sternum – entire length (marked increase in motion) |
Table: Grading of sternal stability on the modified Sternal Instability Scale
Figure: Physical assessment of sternal instability
Sternal Instability Management
Patients diagnosed with sternal instability should be monitored every 3 to 4 weeks. For those with a modified sternal instability scale grade 2 or 3, it is important to notify the medical practitioner to ensure timely medical management such as surgical re-wiring or management of infection.
Patients with sternal instability should continue to follow the “Keep Your Move in the Tube” method to ensure safe performance of transfers and daily tasks.
A medication review may be necessary for those with a dry, non-productive cough secondary to medications (e.g., ACE inhibitors) to minimise risk of sternal instability.
An external bracing system, such as in the figures below, can provide an interim measure prior to surgical repair or to minimise pain and prevent the progression of sternal separation. Early in the post-operative period all women should be encouraged to wear a supportive bra (such as a sports bra or surgical bra) with wide straps and no underwire to minimise stress on the healing sternum and wound.
Figure: Example of external bracing (a. Qualibreath and b. Qualibra)
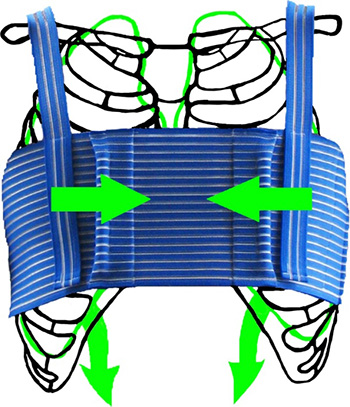
a.
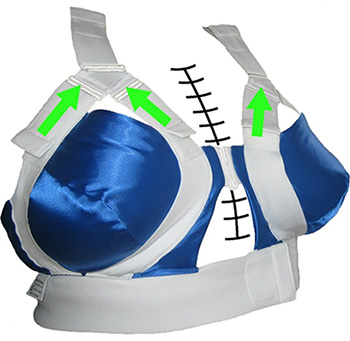
b.
Reproduced with permission from HjorthHealth Pty Ltd, 2012
 Pathophysiology
Pathophysiology
 Treatment & Management
Treatment & Management
 Exercise
Exercise
 Medications
Medications
 Psychosocial Issues
Psychosocial Issues
 Patient Education
Patient Education
 Behaviour Change
Behaviour Change
 Clinical Indicators
Clinical Indicators
 Pathophysiology
Pathophysiology
 Treatment & Management
Treatment & Management
 Exercise
Exercise
 Medications
Medications
 Psychosocial Issues
Psychosocial Issues
 Patient Education
Patient Education
 Behaviour Change
Behaviour Change
 Clinical Indicators
Clinical Indicators
 Pathophysiology
Pathophysiology
 Treatment & Management
Treatment & Management
 Exercise
Exercise
 Medications
Medications
 Psychosocial Issues
Psychosocial Issues
 Patient Education
Patient Education
 Behaviour Change
Behaviour Change
 Clinical Indicators
Clinical Indicators




