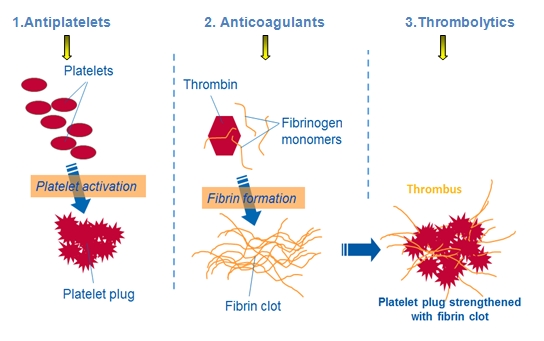
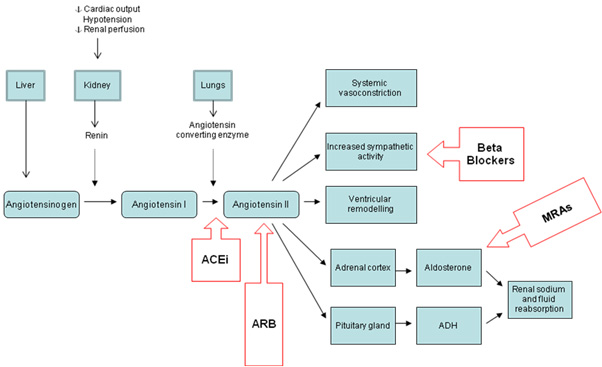
Oral statins (e.g.,atorvastatin, fluvastatin, pravastatin, simvastatin) reduce blood levels of low-density lipoprotein cholesterol (LDL-C), which is a major contributor to atherosclerotic plaque development. They also have other important pleiotropic effects such as plaque stabilisation, antioxidant, anti-inflammatory and antiplatelet effects. These multiple actions mean that statins are used even in ACS patients with normal LDL-C levels and during the acute management phase of ACS.
Statins reduce mortality in patients with NSTEMI and STEMI, and should be routinely prescribed for these patients. They are lifelong medications for patients at high risk of CVD, although the dose may change over time. All patients with ACS are considered 'high risk'.
Nitrates
Nitrates are potent vasodilators. In ACS, nitrates vasodilate the coronary vessels, improving oxygen supply to the heart and peripheral vessels, reducing BP and venous return and, thus, cardiac workload.
Short-acting nitrates such as glyceryl trinitrate (GTN) sublingual tablets or spray are used for symptom control in acute stable angina or ACS. Long-acting nitrates (e.g., GTN patches or isosorbide mononitrate as slow release (SR) tablets) are used to prevent symptoms in stable angina.
Long-acting nitrates have no proven mortality benefits, and their use should be reviewed in patients after percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery where ongoing angina is not expected. These patients should be instructed to seek help immediately if they experience chest pain or ACS-like symptoms.
Morphine
Morphine is used in STEMI, NSTEMI or unstable angina. This opioid is useful for both its analgesic and potential coronary vasodilating effects. Its vasodilating activity is thought to be secondary to decreasing pain and anxiety, which lowers circulating catecholamines and thereby reduces coronary vasoconstriction.
Oxygen
Routine use of supplemental oxygen for acute chest pain is not recommended as there is insufficient evidence supporting its use and also some evidence to suggest potential harm. Supplemental oxygen may increase coronary vascular resistance, potentially leading to poorer outcomes compared to utilising room air.[#ogara-pt-kushner-fg-ascheim-dd-et-al.-2013] Oxygen therapy should only be instituted for hypoxaemic patients (SpO2 <93%) and those with evidence of shock, in order to correct tissue hypoxia.
Oxygen should be used with caution in patients with chronic obstructive pulmonary disease (COPD) and carbon dioxide retention.
 Pathophysiology
Pathophysiology
 Treatment & Management
Treatment & Management
 Exercise
Exercise
 Medications
Medications
 Psychosocial Issues
Psychosocial Issues
 Patient Education
Patient Education
 Behaviour Change
Behaviour Change
 Clinical Indicators
Clinical Indicators
 Pathophysiology
Pathophysiology
 Treatment & Management
Treatment & Management
 Exercise
Exercise
 Medications
Medications
 Psychosocial Issues
Psychosocial Issues
 Patient Education
Patient Education
 Behaviour Change
Behaviour Change
 Clinical Indicators
Clinical Indicators
 Pathophysiology
Pathophysiology
 Treatment & Management
Treatment & Management
 Exercise
Exercise
 Medications
Medications
 Psychosocial Issues
Psychosocial Issues
 Patient Education
Patient Education
 Behaviour Change
Behaviour Change
 Clinical Indicators
Clinical Indicators